8.1 Energy sources
Types of energy sources
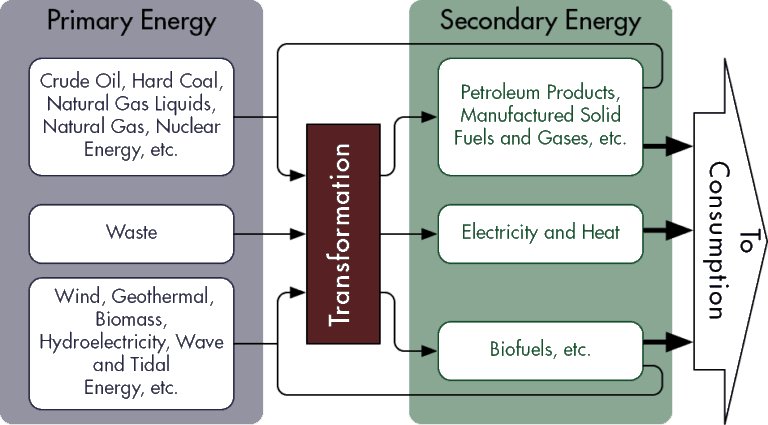
A primary source is one that has not been transformed or converted before use by the consumer. Example: coal burnt in a furnace to convert chemical PE into internal energy of the surrounding.
A secondary source is energy that results from the transformation of a primary source. Example: electricity.
| Renewable energy source | Non-renewable energy source |
|---|---|
| Can be replenished in a relatively short time (human lifetime), or continually generated | Can be replaced only over very long geological times |
| Biomass, solar, wind, hydro (water), geothermal | Coal, oil, natural gas, nuclear |
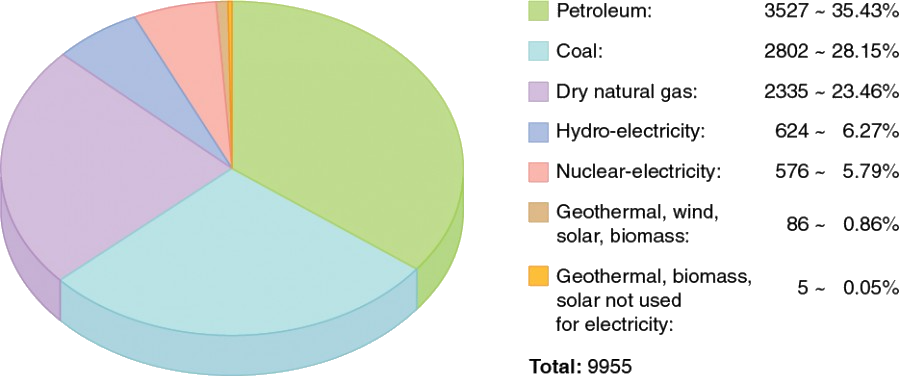 Our world in data - energy use
Our world in data - energy use
Specific energy and energy density
Specific energy is the number of joules that can be released by each kilogram of the fuel.
Energy density is the number of joules that can be released from 1m3 of fuel.
Energy density and specific energy tableSankey diagram
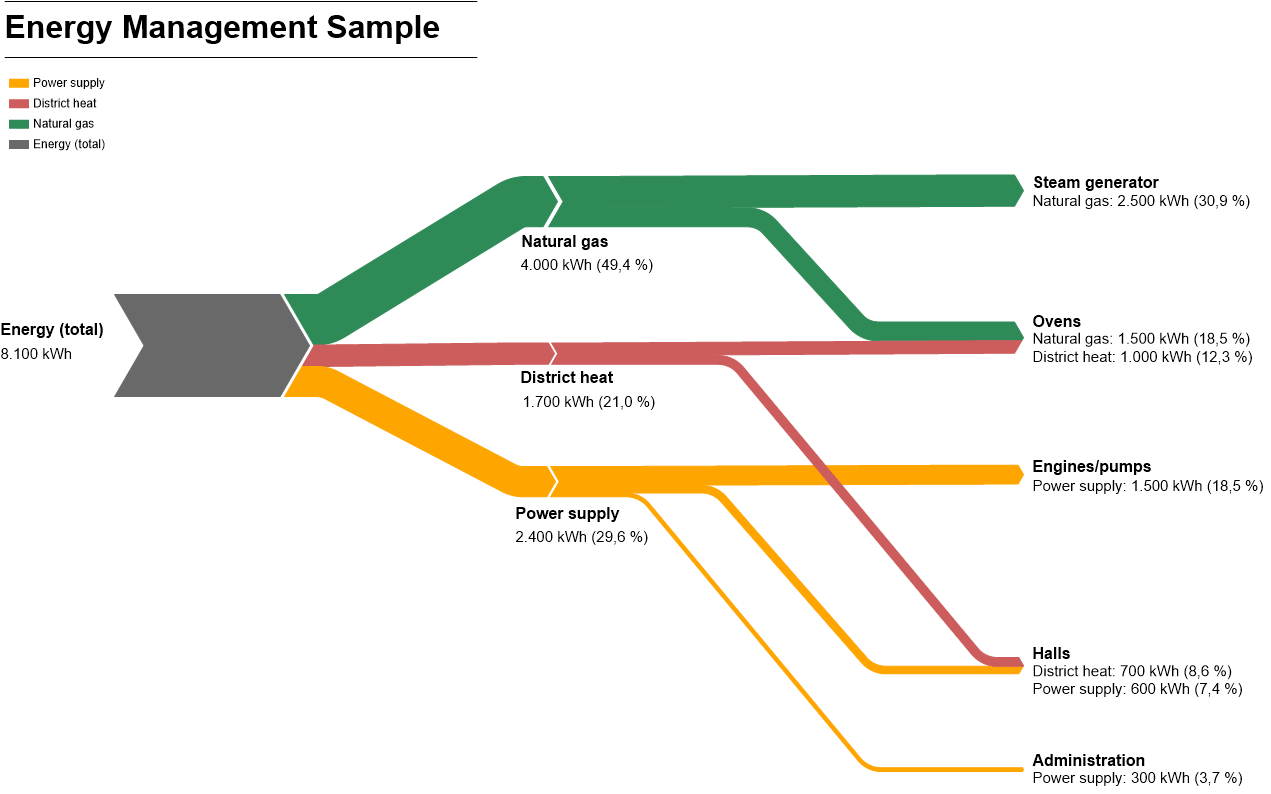
The Sankey diagram is a visual representation of the flow of energy in a device or a process.
Each energy source is represented by an arrow. The width of the arrow is proportional to how much energy it represents.
Power stations
Nuclear power plants

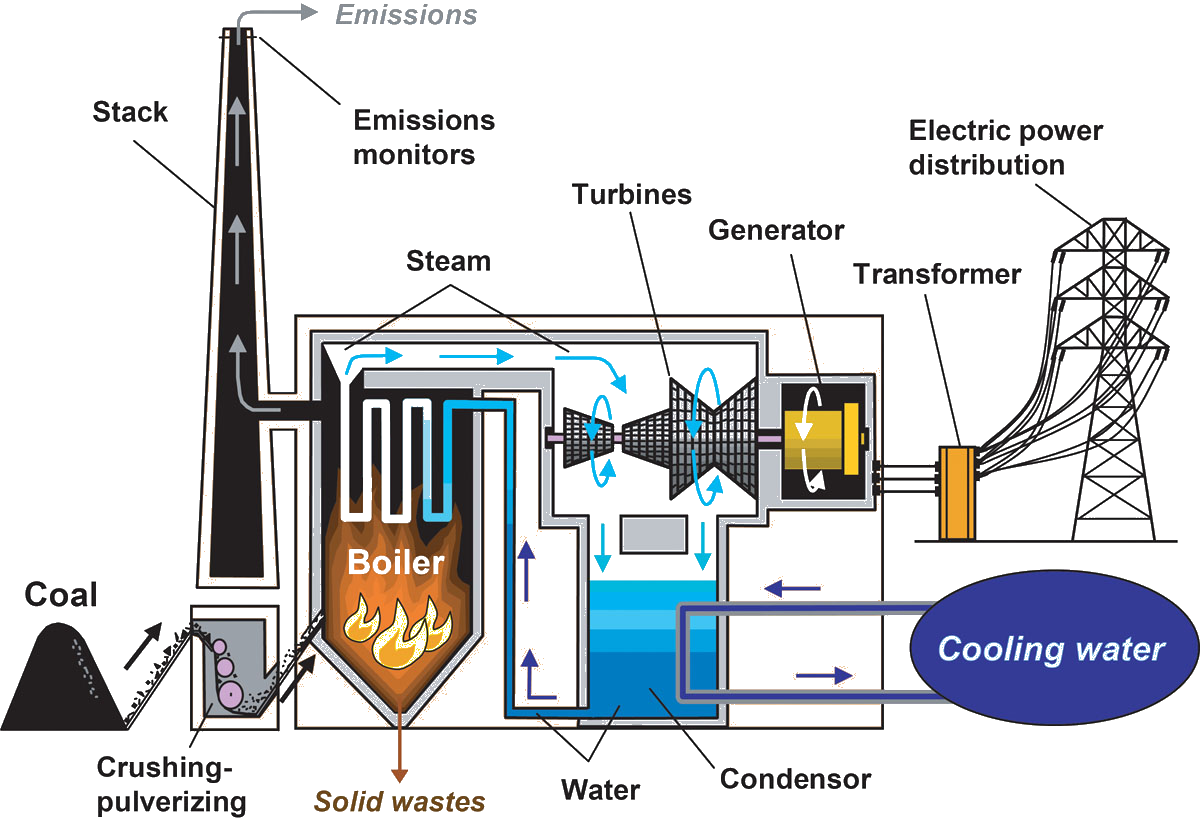
A nuclear reactor is driven by the splitting of atoms, a process called fission, where a particle (a ‘neutron’) is fired at an atom, which then fissions into two smaller atoms and some additional neutrons. Some of the neutrons that are released then hit other atoms, causing them to fission too and release more neutrons. This is called a chain reaction.The fissioning of atoms in the chain reaction also releases a large amount of energy as heat. The generated heat is removed from the reactor by a circulating fluid, typically water. This heat can then be used to generate steam, which drives turbines for electricity production. In order to ensure the nuclear reaction takes place at the right speed, reactors have systems that accelerate, slow or shut down the nuclear reaction, and the heat it produces. This is normally done with control rods, which typically are made out of neutron-absorbing materials such as silver and boron. (World Nuclear Assosiation)
Safety issues
The reactor vessel is made of thick steel to withstand the high temperature and pressure. This also absorbs alpha and beta radiation.
The vessel is encased in layers of concrete that absorbs neutrons and gamma rays.
Nuclear reactor simulatorWhy Chernobyl exploded? (optional)
Inside The Tunnels That Will Store Nuclear Waste For 100,000 Years (optional)
Wind generators
Kinetic energy of the air arriving at the turbine in one second:
The maximum theoretical power:
| Advantages | Disadvantages |
|---|---|
| No energy cost | Variable output on a daily or seasonal basis |
| No chemical pollution | Site availability can be limited in some countries |
| Cost can be high but reduce with economies of scale | Noise pollution |
| Easy to maintain on land | Visual pollution |
Pumped storage

Pumped storage sources use gravitational potential energy of water held at a level above a reservoir is converted to electrical energy, as the water falls to the lower level.
Maximum power from water:
Solar energy
Solar heating panels
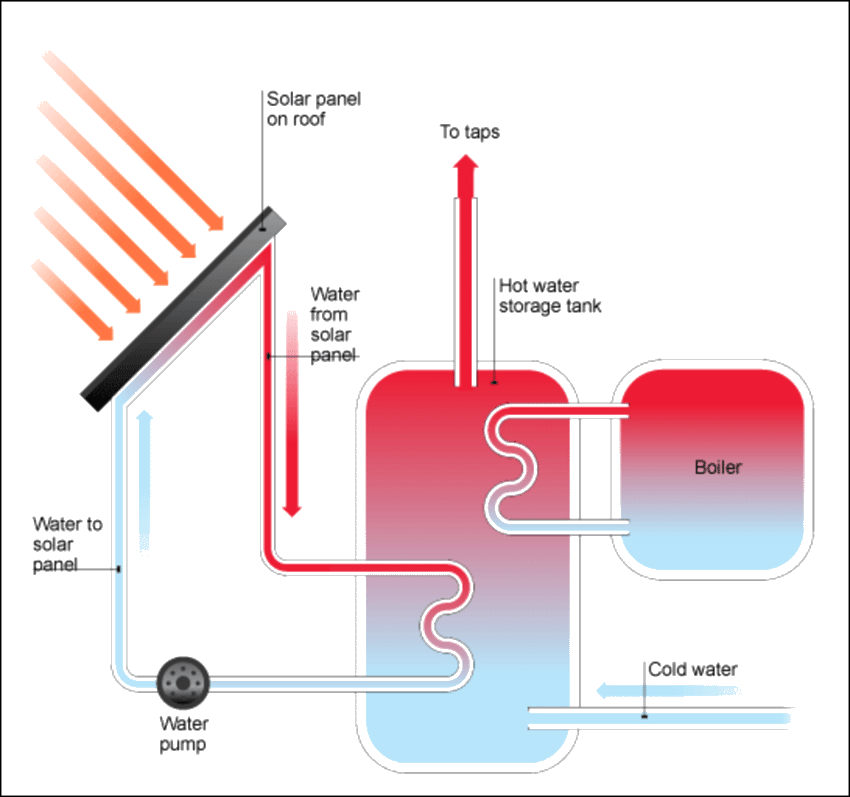
In a solar heating system, a collector (made up of flat-plate PV panels) collects solar energy from the sun. The air or water (or antifreeze) inside a pipe gets warmed up by the heat transferred by the collector. This heat is either carried directly to the interior space by a pump or a venting mechanism, or is stored in a storage system.
Solar water heating history (optional)Solar photovoltaic panels
The photovoltaic materials in the panel convert electromagnetic energy from the Sun into electrical energy. Some materials exhibit a property known as the photoelectric effect that causes them to absorb photons of light and release electrons. When these free electrons are captured, an electric current results that can be used as electricity. (NASA)
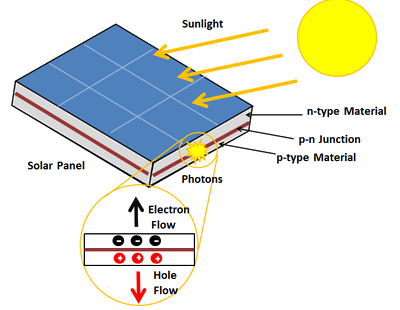
Power converted by the panel:
Written explanation from NASA
8.2 Thermal energy transfer
Conduction, convection and thermal radiation
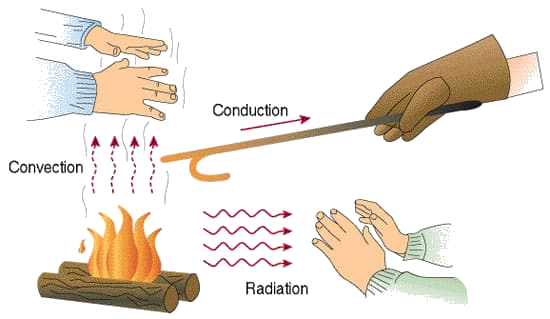
Thermal conduction is the diffusion of thermal energy (heat) within one material or between materials in contact. The higher temperature object has molecules with more kinetic energy; collisions between molecules distributions this kinetic energy until an object has the same thermal energy throughout.
Convection is heat transfer by mass motion of a fluid such as air or water when the heated fluid is caused to move away from the source of heat, carrying energy with it. Convection above a hot surface occurs because hot air expands, becomes less dense, and rises (see Ideal Gas Law). Hot water is likewise less dense than cold water and rises, causing convection currents which transport energy.
Thermal radiation is the transfer of energy by means of electromagnetic radiation. Radiation does not need a medium to propagate. It can travel through a vacuum, as it is a wave.
 Heat transfer video explanation
Heat transfer video explanationSolving the heat equation (optional)
Black-body radiation
Intensity:
All objects with a temperature above absolute zero (0 K, -273.15 oC) emit energy in the form of electromagnetic radiation. A blackbody is a theoretical or model body which absorbs all radiation falling on it, reflecting or transmitting none. It is a hypothetical object which is a “perfect” absorber and a “perfect” emitter of radiation over all wavelengths.
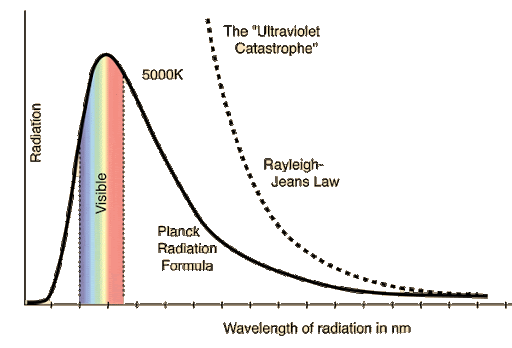
Wien's displacement law:
Stefan-Boltzmann law:
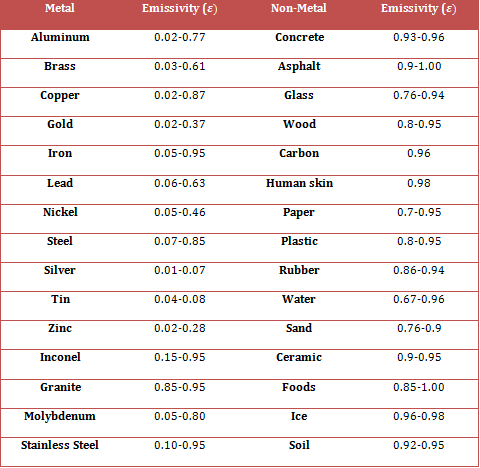
Grey bodies and emissivity
In practice objects can be close to a black-body in behaviour, but not quite 100% perfect. These are called grey bodies.
Emissivity:
 Blackbody Radiation and the Ultraviolet Catastrophe
Blackbody Radiation and the Ultraviolet Catastrophe
Sun and the solar constant
The amount of energy that arrives at the top of the atmosphere is the solar constant.
The solar constant is approximately 1366 Wm-2.
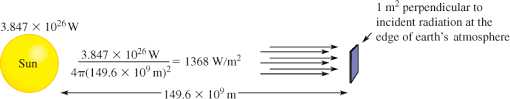
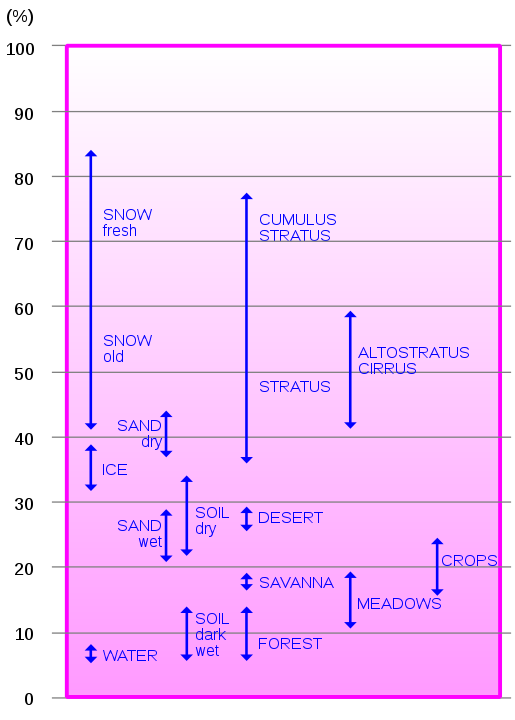
Albedo
When the energy from the sun arrives at ground level, some of it will get reflected by the Earth's surface, as the planet is not a black-body. The extent to which a surface can reflect is albedo (a).
The average annual albedo for the Earth's surface is 0.35, thus 35% of the Sun ray's are reflected back into the atmosphere.
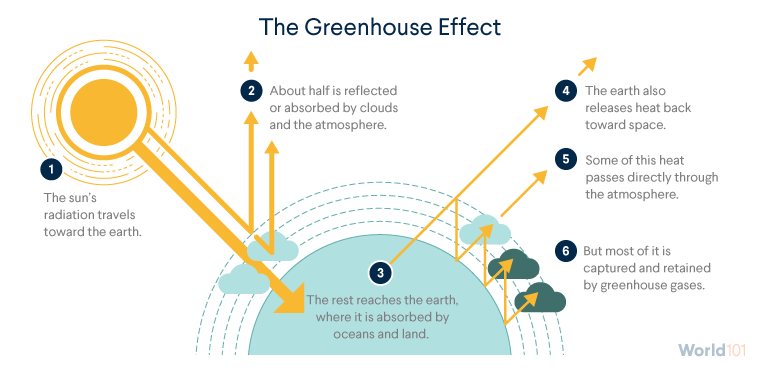
The greenhouse effect and temperature balance
The greenhouse effect is a process that occurs when gases in Earth's atmosphere trap the Sun's heat. This process makes Earth much warmer than it would be without an atmosphere.
The "natural" greenhouse effect is due to the naturally occuring levels of gases.
The enhanced greenhouse effect, in which increased concentration of gases, a result of human industrial processes, increases the Earth's average temperature.
The energies of infra-red photons are much smaller than those of UV, and are not sufficient to break a molecule apart. When the frequency of a photon matches a vibrational state of a greenhouse gas molecule, then resonance occurs.
Each vibrational mode of the CO2 has a characteristic frequency. If the frequency of the radiation matches this, then the molecule will vibrate.
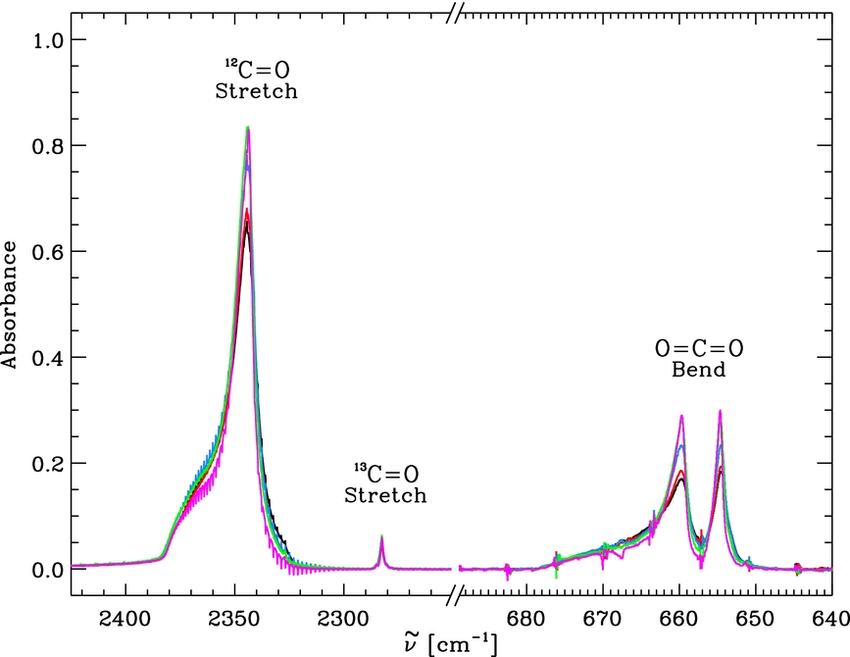
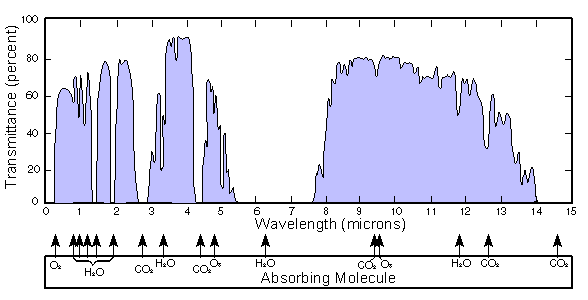
Global warming chain reaction:
Ice and snow covers at the poles melt with increased temperature, which decreases albedo, which increases the rate at which heat is absorbed by the surface.
A higher water temperature, reduces the extent to which CO2 is dissolved in seawater, which increases its presence in the atmosphere and therefore increases the heat absorbed by it.
Global Warming 10113 Misconceptions About Global Warming (recommended)
The energy balance of the Earth
 Interactive energy balance (oversimplification)
Interactive energy balance (oversimplification)
Topic 8 Problems
Number of correct answers: