3.1 Thermal concepts
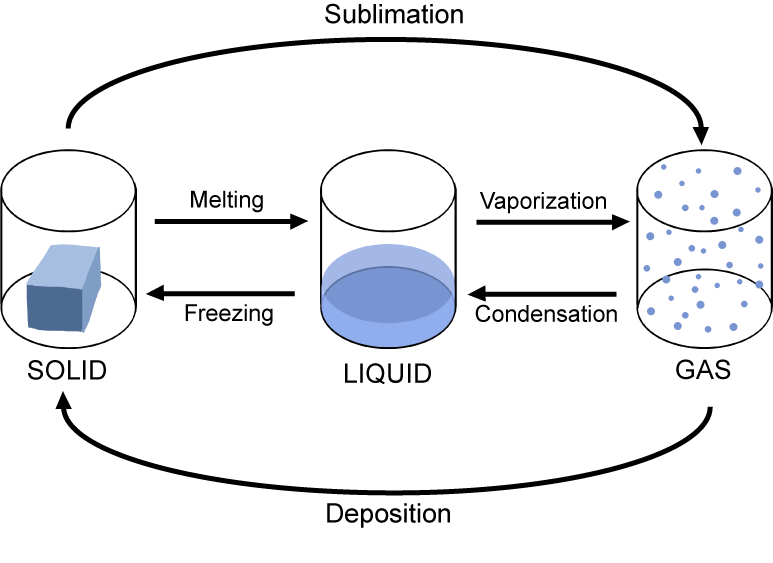
Solids, liquids and gases
Molecules are held together by intermolecular forces

Temperature
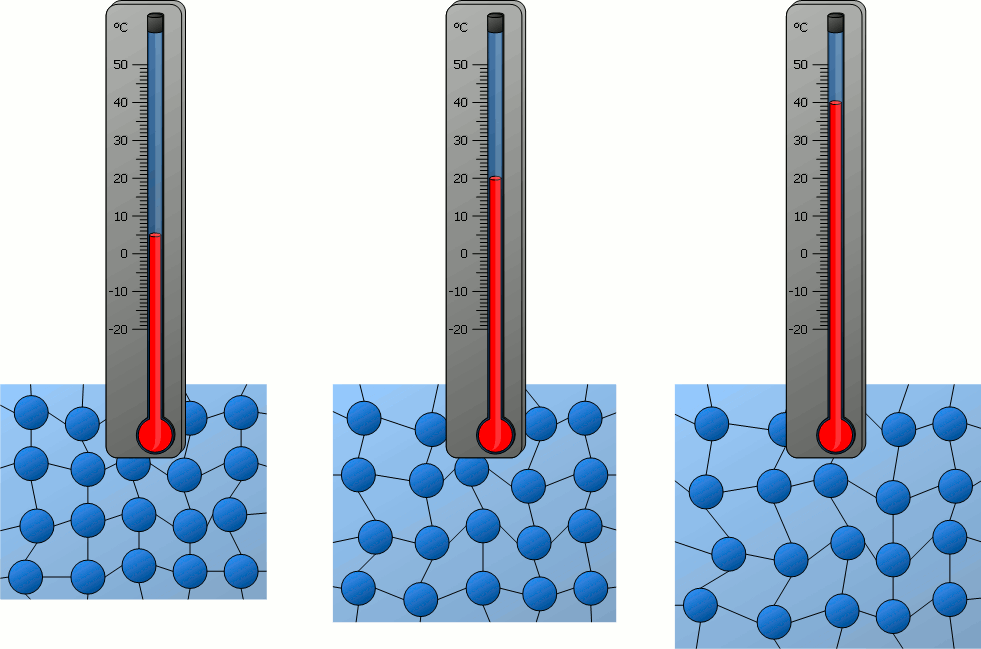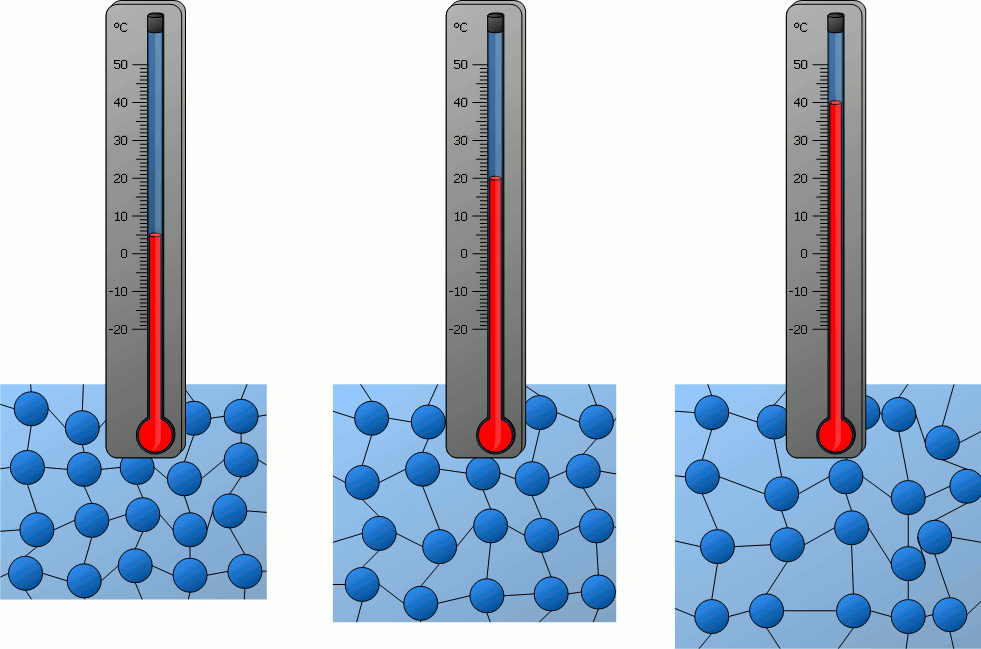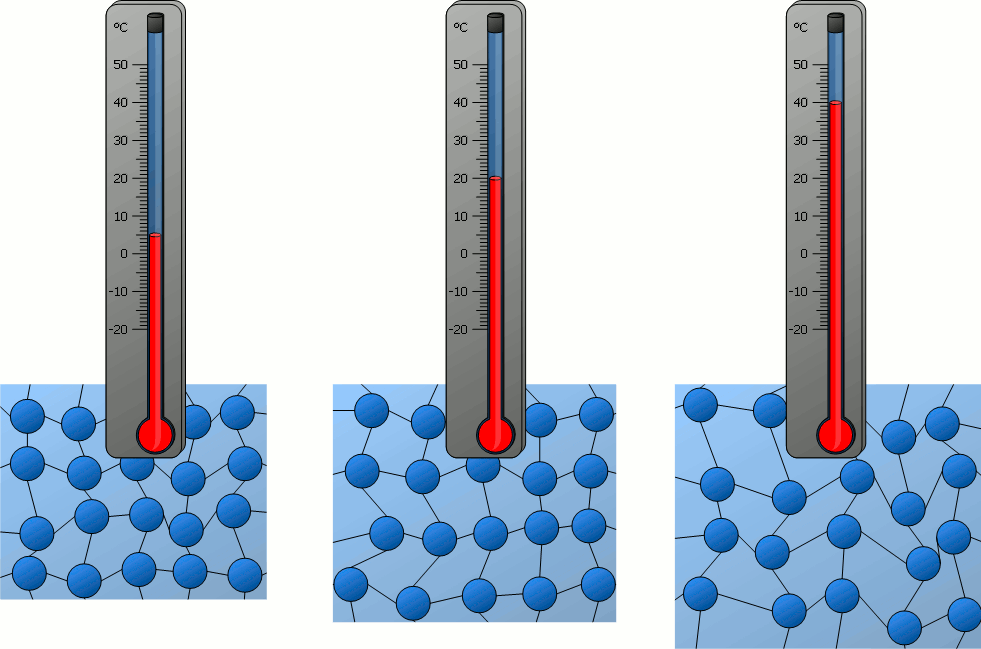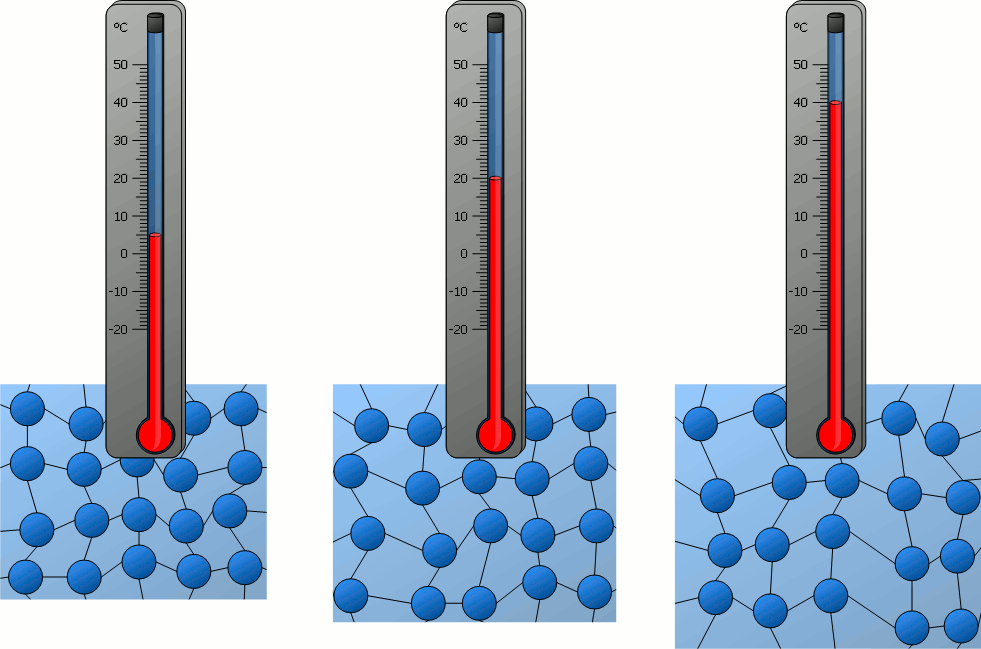
Temperature is a scalar quantity measured in ℃C (Celcius) or K (KelvinBase SI unit), using a termometer.
The energy will tend to pass from a hotter object to a colder object until they reach thermal equilibrium.
 Extra content on heat transfer (not IB required)
Extra content on heat transfer (not IB required)
Absolute Temperature
To find temperature in Kelvin from temperature in Celcius remember that:
Temperature of a body in Kelvin is directly proportional to the average Kinetic energy½mv2 per molecules in the body. Therefore, there cannot exist a temperature lower than 0 Kelvin.
Internal energy
Internal energy is the sum of Kinetic energyassociated with the random/translational rotational motions of molecules and Potential Energyassociated with forces between molecules.
Specific heat capacity
Table of specific heat capacities (Use Isobaric mass heat capacity)
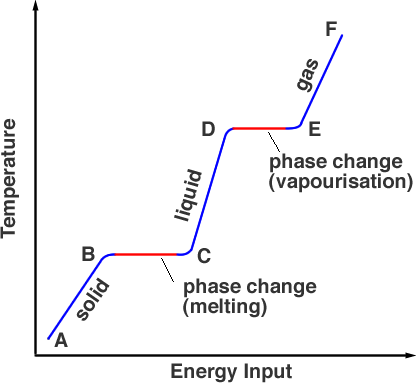
Phase change
| Change of phase | Process | Kinetic energy | Potential energy |
|---|---|---|---|
| Solid to liquid | Melting | Unchanged | Increases |
| Liquid to solid | Freezing | Unchanged | Decreases |
| Liquid to gas | Boiling | Unchanged | Increases |
| Gas to liquid | Condensation | Unchanged | Decreases |
Potential energy increases with temperature because:
1.At a higher temperature, more atoms/molecules are in excited electronic states.
2.At higher temperature, more molecules are in excited vibrational states.
3.At higher temperature, more molecules are in excited rotational states.

Boiling only occurs at boiling point throughout the liquid.
Evaporation happens at any temperature. The particles with highest KE evaporate, reducing average KE.
Specific latent heat
Specific latent heat for fusion: the amount of heat required to change 1kg of a substance from solid to liquid without change in temperature.
Specific laten heat for vaporization: the amount of heat required to change 1kg of a substance from liquid to gas without change in temperature.
3.2 Modelling a gas
Gas laws
Boyle's law
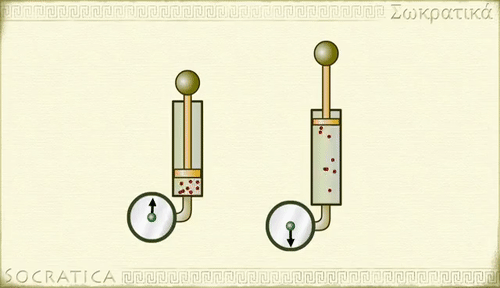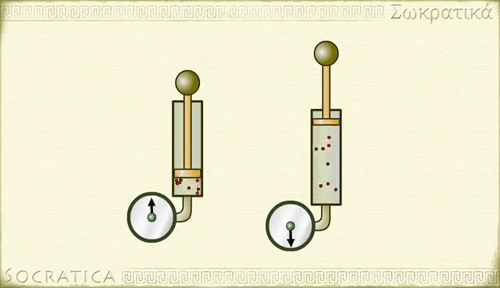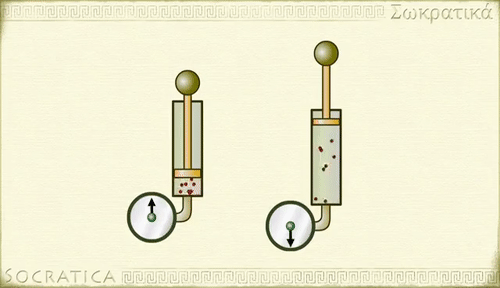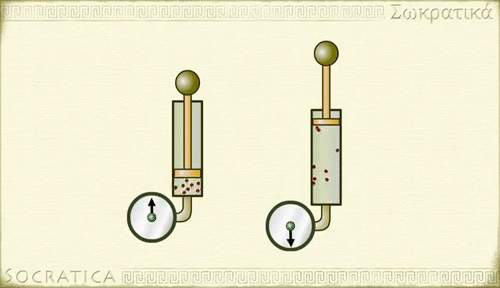
Charles' law

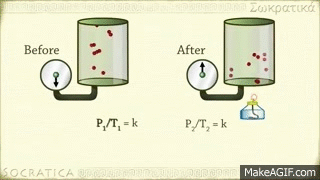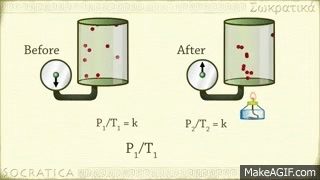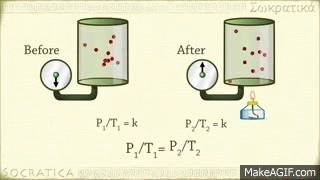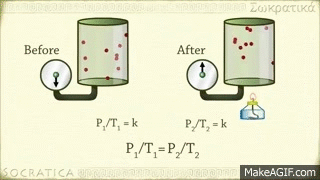
Gay-Lussac's law
Avagadro's law
Combined gas law
Kinetic model of an ideal gas
Ideal gas assumptions:
1.The collisions between molecules are perfectly elastic.
2.The molecules are identical spheres.
3.The volume of molecules is negligible compared to the volume of the gas.
4.Molecules do not interact with each other.
Implications
Absolute temperature is directly proportional to the average KE and average speed of the molecules of an ideal gas.
Mole, molar mass, avagadro constant
Mole
SI measure of quantity of a “chemical entity,” such as atoms, electrons, or protons. It is defined as the amount of a substance that contains as many particles as there are atoms in 12 grams of pure carbon-12. So, 1 mol contains 6.022×1023 elementary entities of the substance.
Molar mass
The molar mass is the mass of a given chemical element or chemical compound (g) divided by the amount of substance (mol).
Real gas vs. ideal gas
There are forces between molecules in real gases.
The volume of molecules is not negligible compared to the volume of gas in real gases.
Real gases are more similar to ideal gases under high temperature and low pressure.

Topic 3 Problems
Number of correct answers: